Cryogenic Electron Microscopy
CNR-IC URT Caserta
Transmission electron cryomicroscopy (cryo-EM) stands as a revolutionary tool in the field of structural biology, enabling scientists to peer into the intricate machinery of life. This cutting-edge technique has revolutionized our understanding of biological macromolecules by transforming our ability to visualize molecular structures at near-atomic resolution (Fig.1). Over the last decade, cryo-EM has found applications across a diverse array of biological systems, offering insights into the architecture of proteins, virus assembly, as well as cellular organization.
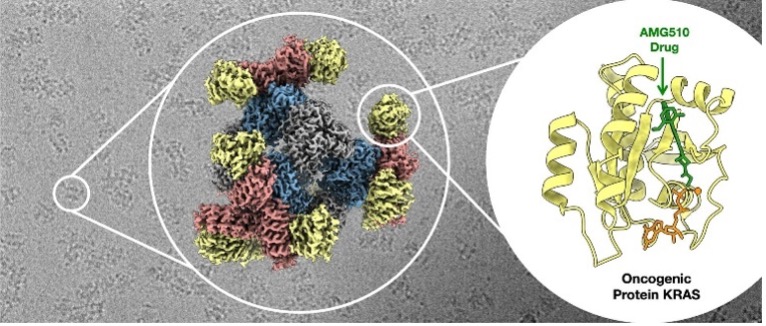
Figure 1: cryo-EM structure of key cancer signaling protein attached to the cancer drug
(Castells-Graells et al., 2023, PNAS | vol. 120 | no. 37)
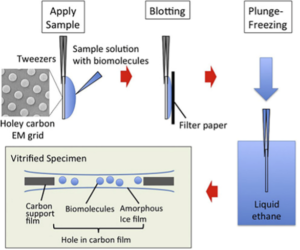
Figure 2: Sample preparation by plunge-freezing
(Murata & Wolf, 2018, BBA – General Subjects, 1862, 324–3)
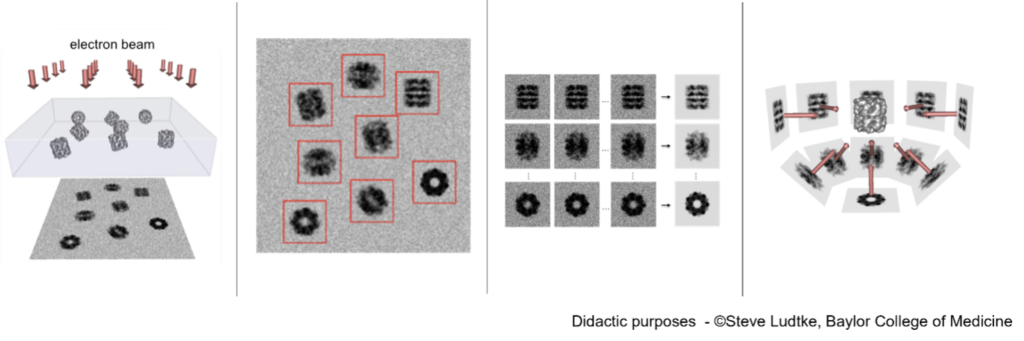
Figure 3: SPA-cryo EM data analysis workflow.
Figure 4: Quality of samples on the grids following the vitrification process.
(© NanoImaging Services)
References:
1. Cheng Y. (2018). Single-Particle Cryo-EM at Crystallographic Resolution. Cell, 171(2), 318–327.
2. Nogales E., Scheres S. H. W. (2015). Cryo-EM: A Unique Tool for the Visualization of Macromolecular Complexity. Molecular Cell, 58(4), 677–689.
3. Bai X. C., McMullan G., Scheres S. H. W. (2015). How Cryo-EM Is Revolutionizing Structural Biology. Trends in Biochemical Sciences, 40(1), 49–57.
4. Kühlbrandt W. (2014). The Resolution Revolution. Science, 343(6178), 1443–1444.
5. Subramaniam S., Kühlbrandt W., Henderson R. (2016). Cryo-EM at IUCrJ: A new era. IUCrJ, 3(Pt 1), 3–7.
Equipment:
Glacios: 200 kV accelerating voltage, field emission gun (X-FEG) source, equipped with an autoloader, ideal for cryo-EM sample optimization and data collection.
Vitrobot Mark IV System: a state-of-the-art instrument widely used for sample preparation. It operates within a controlled environment, maintaining consistent temperature and humidity levels.
Data storage
We use 580TB HD for data storage
High-performance computing cluster
A computing cluster allowing efficient data processing using state-of-the-art data processing software such as Relion or CryoSPARC.
